Uranium hexafluoride
| Infobox on Uranium hexafluoride | |
|---|---|
| Example of Uranium hexafluoride |  |
| Facts | |
| Origin | - |
| Stowage factor (in m3/t) | - |
| Humidity / moisture | - |
| Ventilation | - |
| Risk factors | See text |
Uranium hexafluoride
Description
Uranium hexafluoride (UF6), referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure, is highly toxic, reacts violently with water and is corrosive to most metals. It reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists further reaction.
Application
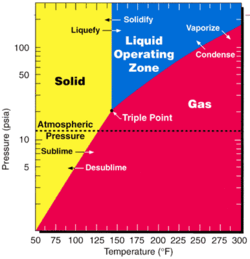
UF6 is used in both main uranium enrichment methods, gaseous diffusion and the gas centrifuge method, because it has a triple point at 64.05°C (147°F, 337 K) and a slightly higher than normal atmospheric pressure. Fluorine has only a single naturally-occurring stable isotope, so isotopologues of UF6 differ in their molecular weight based solely on the uranium isotope present.
All other uranium fluorides are involatile solids which are coordination polymers.
Gaseous diffusion requires about 60 times more energy than the gas centrifuge process; even so, gaseous diffusion nuclear fuel produces 25 times more energy than is used in the diffusion process. Centrifuge produced fuel produces 1,500 times more energy than is used in the centrifuge process.
In addition to its use in enrichment, uranium hexafluoride has been used in an advanced reprocessing method (fluoride volatility), which was developed in the Czech Republic. In this process, used oxide nuclear fuel is treated with fluorine gas to form a mixture of fluorides. This is then distilled to separate the different classes of material.
Shipment / Storage
About 95% of the depleted uranium produced to date is stored as uranium hexafluoride, DUF6, in steel cylinders in open air yards close to enrichment plants. Each cylinder contains up to 12.7 tonnes (or 14 US tons) of solid UF6. In the United States alone, 560,000 tonnes of depleted UF6 had accumulated by 1993. In 2005, 686,500 tonnes in 57,122 storage cylinders were located near Portsmouth, Ohio, Oak Ridge, Tennessee, and Paducah, Kentucky.[12][13] The long-term storage of DUF6 presents environmental, health, and safety risks because of its chemical instability. When UF6 is exposed to moist air, it reacts with the water in the air to produce UO2F2 (uranyl fluoride) and HF (hydrogen fluoride) both of which are highly corrosive and toxic. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated lifetime of the steel cylinders is measured in decades.
Consult the IMDG Code for overseas transport.











